Sodium-ion battery development gathers pace
Sodium-ion batteries could displace lead acid in many applications, according to Professor John Irvine, who leads the joint academic/industrial Nexgenna effort under the auspices of the Faraday Institution to develop the next generation of sodium-ion battery technology (writes Peter Donaldson).
Speaking exclusively to E-Mobility Engineering, he says they will also be able to compete with lithium-ion in terms of energy density and power density while also offering benefits in terms of safety and cost.
Lithium-ion batteries are not the initial target for replacement, however. Prof Irvine believes sodium-ion will replace lead-acid batteries in applications in which cost is paramount before competing with lithium-ion.
Among early target EV applications are small two- and three-wheelers in the developing world, which he describes as a very strong market, along with applications that are less constrained in terms of weight and volume, such as shipping, trains and buses.
“Cost is paramount,” he says. “We are working on the assumption that lithium will be expensive compared with sodium. We can avoid using cobalt, which also is expensive, and are looking to earth-abundant electrodes with manganese and iron as transition metals.”
Another major cost advantage is that sodium-ion batteries can use aluminium current collectors instead of more expensive copper. That is not an option for lithium-ion batteries, because the lithium will react with the aluminium.
Prof Irvine also argues that the use of manganese and iron rather than cobalt and nickel is more environmentally friendly, which is also a consideration for the electrodes.
“On the negative electrode we are tending to use hard carbons rather than graphite, and these are generally biomass-derived carbons.”
Sodium-ion batteries also have a safety advantage over lithium-ion, in that they can be completely discharged, eliminating the risk of explosion during transport, whereas it is very hard to empty lithium-ion batteries.
Prof Irvine says sodium-ion is probably less prone to thermal runaway, but there is more work to be done on that. “It’s still early days. So far, sodium-ion batteries are not performing at the same high current rates as lithium-ion batteries, so they are less likely to go into thermal runaway in that sense.
(Courtesy of the 2021 roadmap for sodium-ion batteries, Nuria Tapia-Ruiz et al, 2021, J Phys Energy 3 031503)
“We would like to be in a situation where we have energy density as high as that with lithium. At the moment, the energy density is certainly competitive, but not with the highest energy density lithium-ion chemistries.
“We need to develop the electrolytes, and that will also help with safety.”
Electrolytes these days tend to mirror the chemistries prevalent in lithium-ion cells but Prof Irvine expects that to change, because the transport of larger sodium ions through an electrolyte is quite different.
“At the moment, we are following current lithium technologies using sodium instead of lithium hexafluorophosphate as the salt, but we anticipate alternative salts to be effective for sodium. That opens the door also to deliver salts that are much safer in terms of chemical hazards.”
He adds that another direction for Nexgenna’s electrolyte r&d is to find solvents that are more stable at higher temperatures while being better suited to sodium, although they are still likely to be hydrocarbon-based.
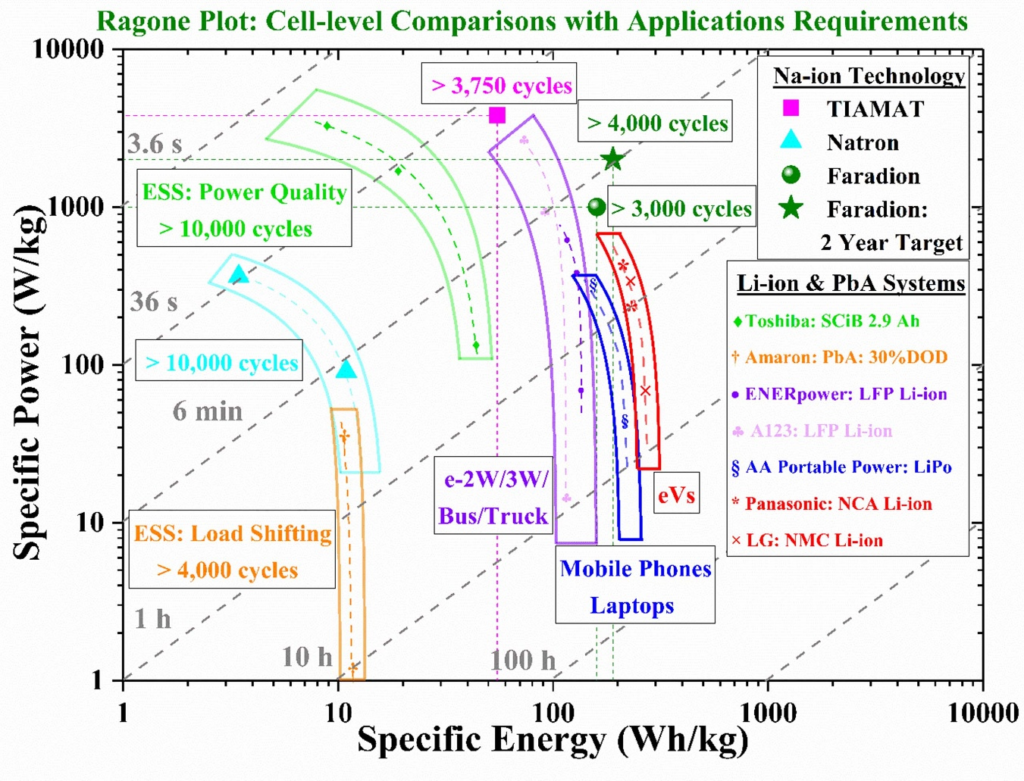
Sodium-ion batteries are making good progress in performance terms. For example, Faradion has achieved about 1000 W/kg in specific power and about 170 Wh/kg in specific energy, according to a Ragone plot in the 2021 sodium-ion roadmap.
At the moment, he Faradion cells deliver energy and power metrics between those of NMC/graphite and LFP/graphite cells, and could therefore be considered as a replacement for most applications where LFP/graphite deployed, the roadmap says.
Outside the Nexgenna effort, Prof Irvine points to rapid progress in China, exemplified by the novel approach of CATL, which launched its first-generation sodium-ion battery in late July. It claims a specific energy of 160 Wh/kg, the ability to charge to 90% capacity in 15 minutes at room temperature and “excellent” thermal stability that exceeds automotive safety requirements.
Despite slightly lower energy density than current LFP batteries, CATL claims major advantages in low-temperature operation and charging speed, especially in extremely low temperatures and high-power applications such as EVs. Capitalising on this, its AB battery mixes sodium-ion and lithium-ion modules in a single pack under the control of a single BMS.
CATL says its second generation of sodium-ion batteries will exceed 200 Wh/kg.
ONLINE PARTNERS