Battery thermal barriers

(Image: Dow)
Bridging the gap
New materials from carbon nanotubes to intumescent polymers are opening up innovative ways of providing thermal management in e-mobility battery packs, as Nick Flaherty finds out
From mica sheets to thermal gap fillers and aerogels, there are now more options than ever for managing heat flow through a battery pack and powertrain, and for mitigating the effects of thermal runaway.
Gap fillers
Thermally conductive gap fillers are designed to fill air gaps between battery cells and cooling systems, providing electrical insulation to the rest of the battery module and assisting in proper thermal management. When a battery includes gap fillers, it is less susceptible to short-circuiting and overheating, both of which can negatively impact overall battery lifespan.
Gap fillers help reduce material usage and waste production by extending the lifetime of the battery pack, and also offer temperature resistance, which allows the battery to operate at different temperatures during charging, discharging and functional use.
Using thermal interface materials such as thermally conductive gap fillers is one way to achieve EV battery performance goals because gap fillers help prevent the battery from overheating. However, common gap filler options can pose certain sustainability challenges. While EVs are fundamentally designed to help reduce environmental impact, these gap fillers can make it difficult for EVs to reach their full potential overall. Therefore, manufacturers should consider alternative options.
While common gap fillers’ attributes help batteries meet end-use performance requirements and can support sustainability initiatives to some extent, their formulation can cause production inefficiencies. For example, many gap fillers are based on aluminium oxide and have a Mohs hardness of nine out of 10. This causes the gap fillers to be abrasive, putting strain on dispensing machines and often causing leaks to the valves. As a result, additional equipment maintenance and downtime are required to address the issue. Other gap fillers come in a solid gap pad format but this also can cause efficiency challenges. Solid gap pad fillers most often rely on manual compression to remove the air or require complex equipment to apply constant pressure. To do this effectively and without error, manufacturers must spend a considerable amount of time ensuring that all air has been removed. If it’s not, then the battery may have poor thermal connection and be more susceptible to overheating, causing safety risks and material waste.
Waste generation
Many traditional gap fillers are based on silicone. While silicone-based gap fillers offer high strength through their thermal and chemical stability, this stability poses battery component disassembly challenges. Silicone-based gap fillers can either leave behind a hard-to-degrade residue or fully render the component unable to be separated down to its constituent parts. This causes the battery component to ultimately be discarded and replaced in full, which contributes to landfill waste generation in addition to increased material usage.
Compared with common options, gap fillers that are based on aluminium trihydroxide have a Mohs hardness of three. These alternative gap fillers are a much lighter and softer liquid material. As a result, they can pass through dispensing machines easily and with little to no abrasion, keeping equipment maintenance to a minimum. Additionally, because they are soft liquids to start, they easily support fast automation processes for air removal. No complex equipment or manual processes to provide constant pressure are needed; instead, they can be immediately compressed and then cured before becoming stable, which streamlines efforts and reduces associated error likelihood as well as associated downtime costs.
Alternative gap fillers are also available in one- and two-component options, both of which help contribute to less waste production compared with other gap fillers. When used in a one-component format, they are oil-based and do not cure. In this case, they maintain the same liquid viscosity throughout their lifespan, which aids easy removability and enhances overall recyclability efforts.
These alternative gap fillers are also available in a two-component format. The two-component format is silyl modified polymer (SMP) based and does cure; however, even so, these gap fillers still maintain a low bond strength. As a result, they are easy to remove when the battery module needs to be repaired, and manufacturers can disassemble the battery module down to its constituent parts and reuse them if needed to reduce overall waste generation.
In addition to streamlining production as well as repair and removal processes, alternative gap fillers possess certain performance characteristics that help enhance overall sustainability. For example, SMP-based gap fillers have inherent flexibility and can crosslink owing to the presence of reactive silyl groups in their molecular structure. This crosslinking begins during or shortly after application and continues to occur within the battery module over the course of the module’s lifetime as it charges and discharges. Therefore, the gap filler continually builds its durability, which can extend the battery’s longevity and increase sustainability.
This repeated crosslinking also allows the gap fillers to form a flexible, elastomeric network with relatively high thermal conductivity owing to the nature of the silyl-based curing reaction and the resulting structure. Furthermore, minimal shrinkage occurs, which helps prevent air gap formation. In addition to reducing the likelihood of overheating and improving overall safety, a battery with low thermal resistance helps extend battery life and optimises charging efficiencies, which both support a more sustainable battery overall.
(Image: Dow)
Aerogels
An aerogel that has been used as an insulation layer in paint is increasingly being adopted in e-mobility battery packs.
This is a synthetic silica powder material that is used as a functional additive. It offers similar material properties as classic aerogels but is more sustainable owing to the raw materials and the simplified – and much less resource demanding – manufacturing process.
The low density and high bulk, low thermal conductivity and high absorption properties are all related to the chemical and structural nature of the complex amorphous structure of intermingled silicate chains. The physical particles consist of around 5% solid material in a network surrounding a great volume of air distributed in countless meso-sized pores.
This uses a patented waterborne process utilising commonly available silica source material, water and green energy. The flexible process allows modification of the materials’ morphology, particle size, porosity and other features, enabling wide usage in many different end products, including e-mobility battery packs. The materials can be chemically modified by surface treatment, by co-precipitation or by mixing with other ingredients to bring additional benefits such as controlled absorption or release.
Phase change materials
Phase change materials (PCMs) are integrated directly into the battery pack structure, often placed between or encapsulating individual cells. When the battery cells generate excess heat, such as during fast charging or high-power discharge, the PCM, which is usually in a solid state, begins to melt upon reaching its phase change temperature. This is an endothermic process, where the material absorbs a large quantity of heat – the latent heat – with only minimal increase in its own temperature. This absorption effectively buffers the cell temperature, preventing localised hotspots and maintaining temperature uniformity across the battery pack.
The critical technical properties of PCMs for EV applications include the phase change temperature. This temperature must be carefully selected to align with the upper limit of the battery’s optimal operating range (e.g., around 50 to 70 C for thermal runaway mitigation, or lower at 45–55 C for general operating temperature control).
A high latent heat capacity is essential because it determines the amount of thermal energy the PCM can absorb per unit mass before its temperature significantly increases. High latent heat allows for effective heat absorption in a compact volume.
However, the thermal conductivity is a major technical challenge. Most organic PCMs such as paraffin waxes are commonly chosen for their suitable temperature range, chemical stability, and affordability, but have inherently low thermal conductivity. This limitation can cause the PCM layer closest to the heat source to melt quickly and become saturated, while the heat struggles to dissipate into the bulk of the PCM material, reducing the overall cooling effectiveness, especially under high-load scenarios.
High material density is preferred for maximum heat storage in a limited space. The change in volume during the solid-to-liquid phase transition must also be managed to prevent leakage and maintain structural integrity. The PCM must also maintain its thermal properties over thousands of charge–discharge cycles, and be non-corrosive and chemically compatible with the battery cells and packaging materials.
To overcome the low thermal conductivity issue, composite PCMs (CPCMs) are developed. These typically involve embedding the PCM within a highly conductive supporting matrix or foam, such as expanded graphite, carbon fibre or metal foams. These additives can significantly enhance the effective thermal conductivity, sometimes by an order of magnitude, allowing for faster heat transfer away from the battery cells and quicker ‘recharging’ (re-solidification) of the CPCM during resting periods or when other cooling methods are active.
Another technical integration involves combining the passive cooling of PCMs with an active system such as liquid cooling. The PCM acts as the primary buffer against temperature spikes and thermal runaway propagation, while the liquid-cooling system is responsible for removing the accumulated heat from the solidified PCM and rejecting it to the environment.
They are effective at reducing peak temperatures and improving temperature uniformity across the cell array, thereby prolonging battery life and enhancing performance.
The main technical hurdle remains the low thermal conductivity of organic PCMs. Other challenges include the additional weight of the PCM material (although it is often lighter than complex active systems), the need for robust encapsulation to prevent leakage upon melting and the fact that a PCM system can store only a finite amount of heat before it’s fully melted, thereby requiring an external heat rejection mechanism for continuous high-load operation.
Carbon nanotube pads
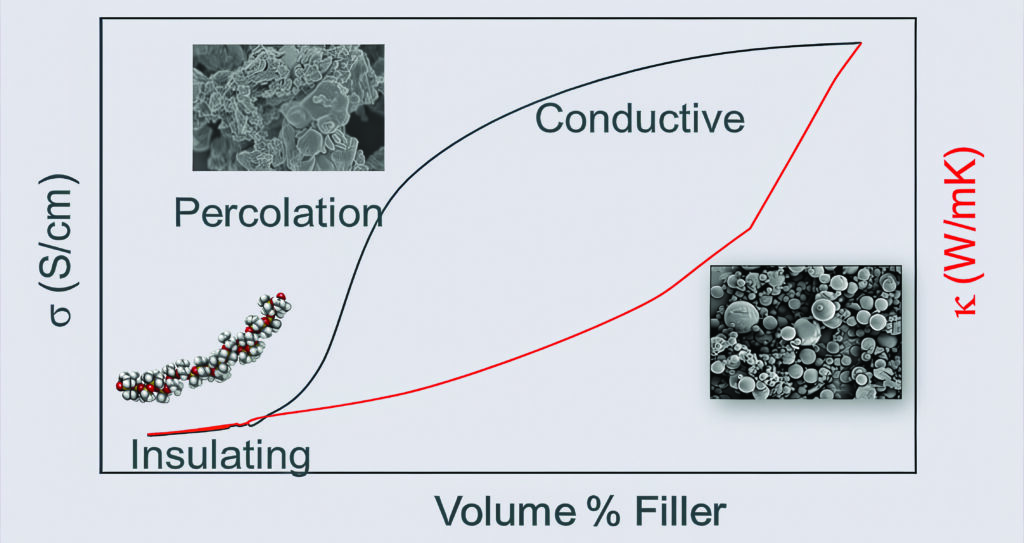
A new approach is to combine very stable materials such as carbon nanotubes (CNTs) with silicones to create thin, thermally conducting pads that have a number of advantages.
Dispensable silicone materials are well established for thermal interfaces because they have the ability to withstand high temperatures and can be loaded with different conductive fillers – both thermal and electrical – and are easy to dispense.
These silicone-based gap fillers and adhesives can provide a thermal density of 1–10 kW/m2 through the combination of the filler type and the loading.
However, there is increasing demand for thermally conducting pads to offer an alternative to PCMs with additional advantages. Silicone pads and silicone adhesive pads are suitable if the surface is not smooth and are used specifically where there is no pump out, i.e., where the dispensed material migrates under increased temperature.
Elastomer pads are typically thick at around 2 mm, while CNT pads are typically 90–150 μm, allowing the heatsink to be as close as possible to the heat source. The pads are reworkable and can be reapplied, making repairs and recycling much easier compared with silicone dispensing. This is a key advantage for end-of-life disassembly of the battery pack and the powertrain.
An advantage of vertically aligned CNTs grown from an aluminium core is that the core creates a very significant XY distribution of heat. This feature can be used for localised heat distribution or in a larger pad across a larger surface area.
However, use of these thinner pads must be designed into the pack from the start to take advantage of the horizontal heat removal, rather than using them as a drop-in replacement for other pad approaches.
Mica sheets
Mica, or silicate, sheets are used predominantly for their superior properties as a thermal barrier and an electrical insulator rather than for conducting heat away during operation. This dual functionality is critical for safety and thermal runaway propagation mitigation.
Mica’s primary thermal role is not to conduct operational heat but to isolate heat generated during catastrophic failure. The thin mica sheets can also be used to provide dielectric isolation between individual cells and modules, high-voltage busbars and structural components, and the battery pack and vehicle chassis.
The material has relatively low thermal conductivity, especially compared with that of typical gap fillers. This characteristic is intentionally used to slow the lateral heat transfer between adjacent battery cells or modules. In the event of one cell entering thermal runaway, the mica barrier provides a delay before the high heat and fire can propagate to neighbouring cells, offering a crucial safety window for passengers to exit the vehicle.
Phlogopite mica exhibits extremely high thermal stability, maintaining structural integrity and insulating properties at temperatures up to 1200 C. This stability is essential because during a thermal runaway event, the released gases and flames can reach these extreme temperatures. Mica sheets act as a flame and heat barrier that resists burnout and disintegration.
The sheets are often enhanced with resin binders, allowing them to withstand the high pressure and impact from ejected molten material and shards that a failing battery cell can release at high velocity.
Mica is also one of the best naturally occurring electrical insulators, making it a vital component for high-voltage battery systems. The breakdown of 20 kV/mm is crucial because EV battery voltages increase from 400 to 800 V and beyond.
By preventing electrical contact, especially in dense battery packs, mica ensures the safety and reliability of the high-voltage system and prevents secondary failures that could be triggered by internal arcing or short circuits.
The sheets are used in various forms, including rigid plates between modules, as flexible sheets or tapes to wrap busbars and wire harnesses and as die-cut gaskets for specific clearances. The material can be easily cut and shaped to required dimensions, even to thicknesses as low as 0.1 mm, providing a balance in the requirements for thermal protection, electrical insulation and weight minimisation.
Intumescent polymers
Another increasingly popular approach in the design of battery packs, especially those used in EVs, is to use an intumescent polymer. This is a polymer that, when subjected to heat or fire, undergoes a chemical transformation that causes it to expand significantly, forming a charred, insulating layer. This charred layer serves to protect the underlying material by reducing the transfer of heat and acting as a barrier to flames, thereby effectively isolating individual cells or modules, and preventing the spread of heat and fire from one cell or module to another.
Insulating char formation
The char layer formed by intumescent polymers acts as an insulator, which reduces heat transfer. This is critical in battery packs because excessive heat can cause adjacent battery cells to enter thermal runaway. By isolating and insulating cells, the char can prevent or slow this chain reaction.
The insulating layer formed by intumescent polymers can help contain flames and potentially harmful gases. This is particularly important if a cell vents or releases gases, which can be both toxic and flammable. By controlling the escape pathway of these gases, the risk of explosion or fire spreading is minimised.
In applications like EVs, weight and space are critical considerations. Intumescent polymers provide an efficient fire barrier without significantly increasing the weight or size of the battery pack.
Intumescent polymers can be applied as coatings, incorporated into casings or used as separators, allowing for flexibility in battery design and optimisation of fire-protection measures. Compared with other passive fire-protection methods, intumescent polymers can be more cost-effective over the product’s life cycle owing to their relatively inexpensive raw materials, simple application processes, minimal maintenance requirements and ability to integrate seamlessly into existing manufacturing lines. Their efficiency in fire protection can also lead to savings from reduced collateral damage, regulatory compliance and potential reductions in insurance premiums.
Process compatibility
Intumescent materials can be integrated into current manufacturing processes without need for substantial changes, making their adoption easier.
Given the increasing energy densities of modern batteries and the associated risks of thermal runaway and fires, integrating intumescent polymers into battery pack designs offers an added layer of safety, helping protect both the equipment and the users.
Unlike mica or aerogel, which must be cast, cut, stacked and taped into position, intumescent thermoplastics can be injection-moulded, over-moulded or extruded into three-dimensional shapes.
In a high-voltage EV pack, copper busbars sit only millimetres from live terminals. An arcing fault can reach 1500 C in microseconds. Typical engineered plastics can create conductive gases when they degrade, which can lead to arcing within a battery pack, similar to lightning in a volcanic eruption. An injection moulded wrap-around busbar cap has high dielectric strength, which can deter arcing that could start a thermal runaway event or ignite other plastics in the pack.
The composites include a high loading of hydrated minerals that decompose to release water vapour to dilute the flammable gases in and/or around battery packs. The surface material undergoes phase change to shield critical components leaving a reserve of insulative material underneath.
The intumescent reaction starting at 200 C triggers an endothermic reaction creating fire-resistant properties of the material with an expansion ratio to two to three times. This isolates areas that are in thermal runaway by creating thermal separation and producing an insulating layer against fire.
There are many options for thermal interface materials in e-mobility systems. From mica sheets used to isolate cells and modules in the event of thermal runaway, to intumescent polymers that expand and char to protect the pack, and there are more innovations emerging designed to protect the system. Existing thermally conductive materials are evolving, with silicones combined with CNTs to produce thin pads that can be used in difficult environments and enable boost reusability.
Acknowledgements
With thanks to Gifford Shearer at Dow , Alexander Grahn at Svenska Aerogel and Sean Luo at Pyrophobic Systems.
Some suppliers of battery thermal barriers
3M
AIS
Aspen Aerogels
Bostik
Carbice
Dow
Electrolock
Henkel
Kulr Technology
LG Chem
Morgan Advanced Materials
Oerlikon
Parker Lord
Pyrophobic Systems
Rogers Corporation
Saint-Gobain
Svenska Aerogel
Thermulon Aerogels
Click here to read the latest issue of E-Mobility Engineering.
ONLINE PARTNERS